Pharmaceutical delivery devices undergo extensive laboratory testing to ensure that they operate as designed and are safe for patients to touch and use. This may
include E&L, etc. But the testing doesn’t end there. Transporting the device from one location to another requires strategy and care to ensure that no damage occurs that could impact the proper function of the device.
However, not all transportation journeys are created equal. Some transportation undertakings pose very little risk to the pharmaceutical delivery device, whereas others are more hazardous. Laboratory testing can offer tremendous insight when devising a strategy for shipping pharmaceutical delivery devices via more hazardous transit channels, however it may be unnecessary for certain stages of the journey.
A risk analysis matrix is an excellent tool for determining the risk level for a given transportation scenario based on multiple variables. This allows manufacturers to be strategic with their resources when making decisions about laboratory testing.
A risk analysis matrix assigns risk to different levels of a given variable, such as process step, type of distribution cycle, historical data, or the nature/scope of the packaging. It then compares it to a second variable to determine overall risk and then determines next steps. The below chart analyzes risk based on the process step and the type of distribution cycle.
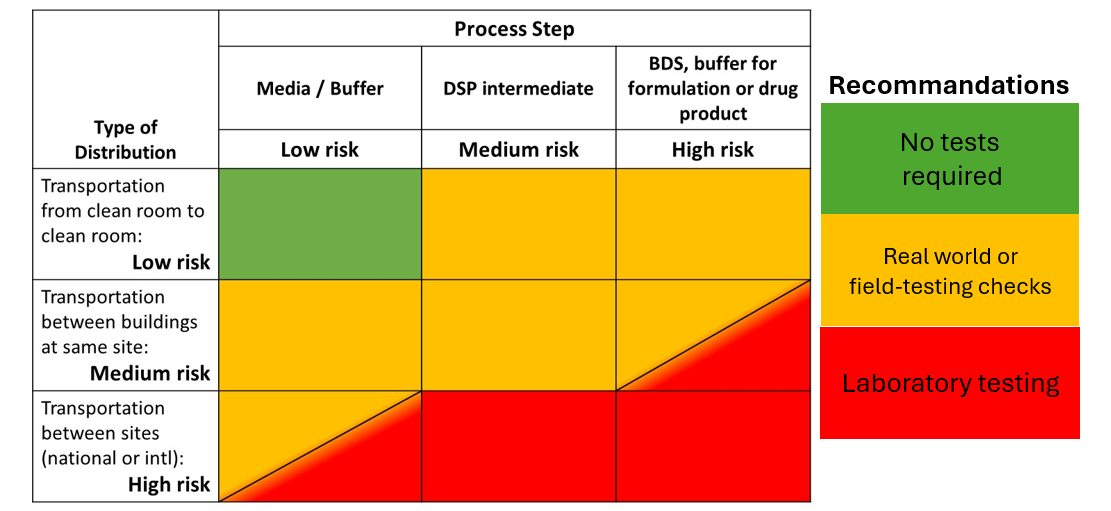
In some low-risk cases, testing of any kind is unnecessary. For other scenarios, field testing or “real world” testing, is sufficient. To conduct a field test, a sample package is sent through its intended supply chain without any special modifications. When the sample arrives at its destination, the package and the contents are examined for damage or failure.
Field testing is simple to execute. No special equipment is needed, and it can be conducted at any time. However, there are two primary drawbacks:
1. Time
Navigating a long supply chain can take days or even weeks, forcing manufacturers to wait a long time for results.
2. Ambiguity
If a sample package arrives damaged, it can be difficult to determine the exact cause or combination of causes. This can make it challenging for manufacturers to redesign the package, since they don’t know which hazards to account for.
In high-risk scenarios, or when field testing falls short, the best strategy is laboratory transit testing. Laboratory distribution testing simulates the hazards of the supply chain in a controlled environment, allowing manufacturers to predict package performance before deployment. Every step of the testing process can be monitored in real time, so manufacturers can understand exactly when and why damage occurred and devise a plan to address it through a redesign if necessary.
Laboratory distribution testing methods fall into four broad categories:
• Climatic conditioning
The goal of climatic conditioning is to evaluate how a packaging system will respond to certain types of hazards, such as compression, vibration, or shock, under certain environmental conditions. The test sample is exposed to extreme temperatures, humidity, atmospheric pressure, or a combination before undergoing additional testing. For example, plastic packaging may be placed in a cold chamber before testing to see if the materials are vulnerable to becoming brittle in cold-weather climates.
• Stacking or compression testing
Compression testing allows manufacturers to make decisions about how many packs or pallets can be stacked in a truck or warehouse by observing the effects of stacking on their packaging system.
• Shock testing
A package may be exposed to a wide variety of shocks throughout the supply chain, from being dropped by a warehouse employee to the jolt of a forklift making contact with a large shipping container. Shock testing can recreate these and many other shock events in a controlled environment.
• Vibration
Trucks, trains, planes, and virtually every other mode of transportation exposes packaging to vibration, though the intensity and frequency varies. Specific vibration profiles, developed from aggregated field data, can be introduced in a laboratory environment and even accelerate the effects of a long-duration journey.
Many ASTM, ISO, and ISTA test specifications combine these methods to qualify packaging systems. The test methods most often used to test packaging for pharmaceutical delivery systems are:
 Please note that not all of these methods are recognized by the FDA.
Please note that not all of these methods are recognized by the FDA.
In some scenarios, a standardized test may not be suitable for qualifying certain devices, packaging systems, or supply chains. When this happens, an experienced distribution testing laboratory can design a custom testing protocol for the unique situation. Testing professionals can combine multiple test methods and variables to recreate specific environments in a controlled environment, granting manufacturers deeper insight into how their device or packaging system will respond to the supply chain, so they can make confident decisions about next steps.
The team at Smithers has years of experience designing and conducting transit testing protocols for pharmaceutical delivery devices. We understand the challenge of shipping a fragile medical device through potentially harsh conditions and can help you design a packaging system that protects your device and ensures intact delivery.
To learn more about our capabilities,
talk to a member of our team

 Please note that not all of these methods are recognized by the FDA.
Please note that not all of these methods are recognized by the FDA.
