ISO 13485: The Latest Revision
Find out what the BioOhio panel of experts had to say about the latest revision of ISO 13485:2016.
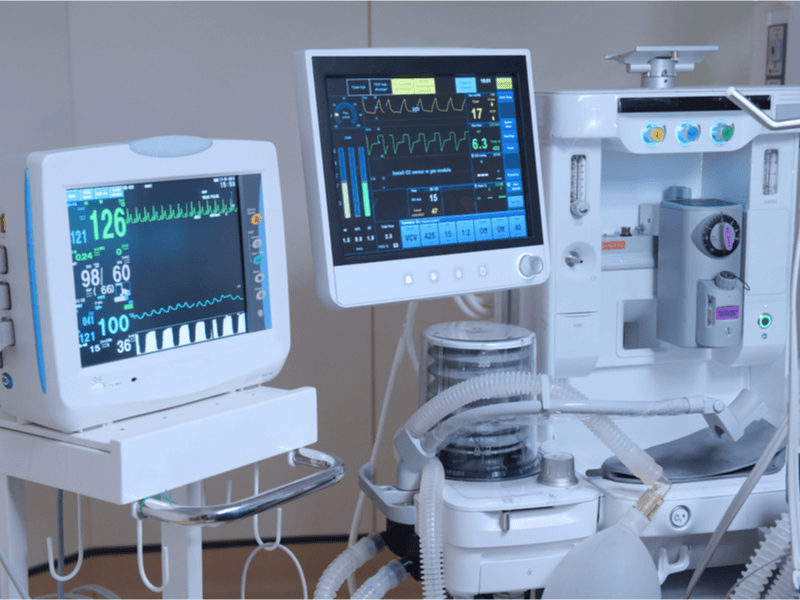
According to the World Health Organization, medical devices are essential for safe and effective prevention, diagnosis, treatment, and rehabilitation of illness and disease. That’s all pretty straightforward, but it’s the next sentence that stands out:
“The achievement of health-related development goals, including the Millennium Development Goals upon proper manufacturing, regulation, planning, assessment, acquisition, management and use of medical devices which are of good quality, safe and compatible with the settings in which they are used.”
What the WHO is stating in the above is the importance of quality throughout the entire lifecycle of the “management and use” part of medical devices.
This is where ISO standards come into play. For example, there is a specific ISO standard, ISO/TC 121/SC-3, which focuses on the safety and essential performance of the aforementioned respiratory devices (ventilators) used for patient care. But, this a particular example for a very specific type of classification of device and not applicable to other types of medical devices, but it’s a good instance of how granular and supportive ISO can be. In a broader, more all-encompassing sense, ISO 13485:2016 “..is designed to be used by organizations involved in the design, production, installation, and servicing of medical devices and related services.” (ISO.org).
This includes designers, component manufactures, assemblers, repair services, and so on. Why is ISO 13485:2016 the go-to when it comes to medical devices? According to ISO.org:
“Regulatory requirements are increasingly stringent throughout every step of a product’s life cycle, including service and delivery. Increasingly, organizations in the industry are expected to demonstrate their quality management processes and ensure best practice in everything they do. This internationally agreed standard sets out the requirements for a quality management system specific to the medical devices industry.”
During this time of crisis, Smithers has received requests from medical device component manufacturers to receive ISO 13485:2016 certification services expediently, as they produce parts that are crucial to the device's build and production. Ultimately, getting more needed medical devices out into the field where they are needed most.
Now, more than ever, we are honored to play our part in this process and are determined to execute every step with the highest care. If your business is facing challenges related to medical device certification, specifically ISO 13485:2016, we’re here to help. We are striving to support our clients through COVID-19 and beyond, however, we can.