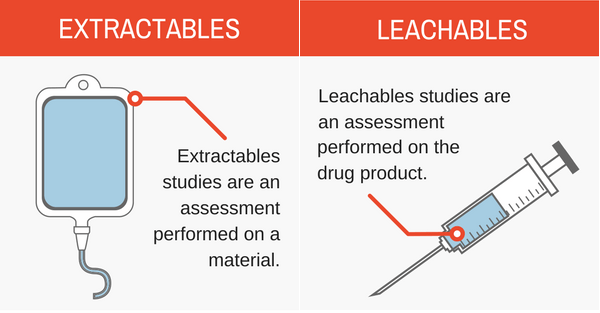
Extractables and leachables studies enable manufacturers to identify, quantify and identify the risk of leachable impurities migrating into a drug solution from container closure systems, processing equipment or packaging. This makes the successful examination of extractable and leachable substances extremely important for both the protection of patients and adherence to regulatory expectations.
Global Expertise
Smithers offers chemical analysis laboratories in both the UK and US, offering a comprehensive range of extractables and leachables testing techniques. We also offer primary pack testing, distribution studies, bioanalytical services and biocompatibility extractables testing.
Smithers provides extractables and leachables testing on a wide range of closure and drug delivery systems, including:
- Biopharmaceuticals / Biologicals
- Pre-filled syringes
- Parenteral and Ophthalmic products (PODPs)
- Orally inhaled and nasal drug products (OINDP)
- Container / Closures
- Single Use Systems and Processing Equipment
- Medical Devices
- Combination Products
- Electronic Nicotine Delivery Systems (ENDS)
Regulatory Compliance
Our experts conduct extractables and leachables assessments in accordance to regional guidance and regulatory specifications, including United States Pharmacopeia (USP), FDA, MHRA and EMA requirements. We also undertake extractables and leachables studies to PQRI recommendations and BPOG guidelines.
We can provide an extractables and leachables analysis to demonstrate product and material compliance with:
- USP <1661> Evaluation of Plastic Packaging Systems and Their Materials of Construction with Respect to Their User Safety Impact
- USP <1663> (Assessment of Extractables Associated with Pharmaceutical Packaging/Delivery Systems)
- USP <1664> (Assessment of Drug Product Leachables Associated with Pharmaceutical Packaging/Delivery Systems)
- USP <232> (Elemental Impurities)
- USP <381> (Elastomeric Closures for Injections)
- USP <661> (Containers – Plastic)
- USP <661.1> Plastic Materials of Construction
- USP <661.2> Plastic Packaging Systems for Pharmaceutical Use
- USP <665> Plastic Components and Systems Used in Pharmaceutical Manufacturing
- USP <671> (Containers – Performance Testing)
- BPOG single use extractables studies
We will work with you to ensure that your regulatory requirements are fulfilled by the testing programme.
Techniques
Numerous analytical techniques are used for extractables and leachables studies because no single analytical technique can detect all of the potential contaminants. The techniques used typically include:
- Gas Chromatography Mass Spectrometry (GC-MS)
|
- Liquid Chromatography Mass Spectrometry (LC-MS)
|
|
|
|
- Non-Volatile Residue (NVR) and Fourier Transform Infrared (FT-IR) spectroscopy
|
- Total Organic Carbon (TOC)
|
- Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES)
|
|
|
Custom Extractables and Leachables Study Programmes
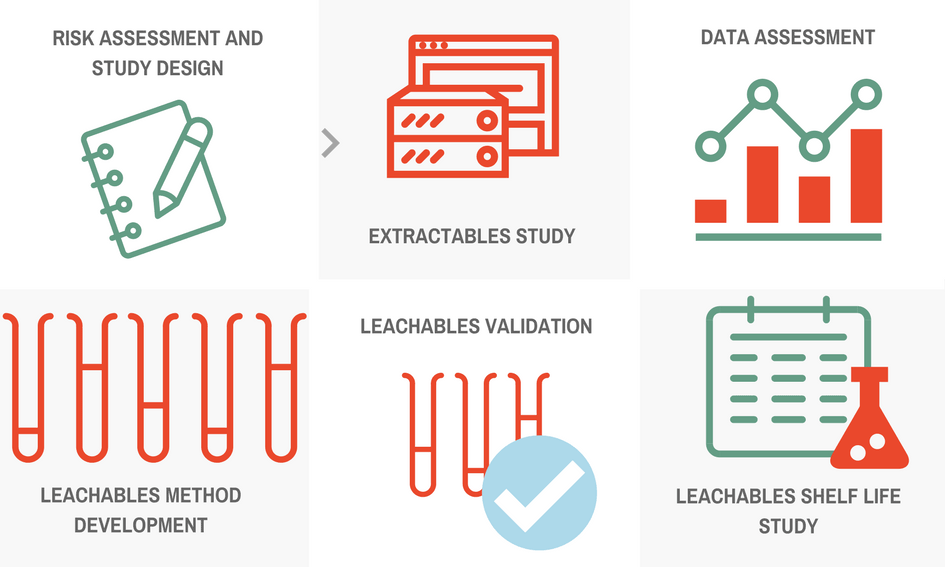
Our experts will design an extractables and leachables study tailored to your project’s requirements, using the following extractables and leachables framework:
We have an open door policy, and clients are welcome to visit our site.
Comprehensive Risk Assessments
Before undertaking an extractables and leachables analysis, our experts will make a comprehensive risk assessment of your product or device. This could include various factors - depending on your product - such as daily dose, administration route, toxicity classification, material contact, dosage form, patient population, duration and construction materials.
This assessment gives our clients the ultimate assurance that our testing will address the relevant areas of concern for their project, as well providing the transparency of our rationale and subsequent approach.
Recycled Materials Support
The experts at Smithers can help you evaluate recycled or post-consumer recycled (PCR) materials for your medical and pharmaceutical products. Our team has years of experience performing investigative extractables and leachables assessments and evaluations to ensure confidence in your materials and products.
Analytical Expertise
Smithers has a 90-year history of working with rubber and plastics, and over this time we have development in-house GC-MS, LC-MS and FT-IR library database of thousands/hundreds of substances to aid in the identification of E&L substances. We are well-placed to assist you in the identification of unknown leachables with our E&L studies.
Expert Support
Our experts can provide consultative support at every stage of the extractables and leachables process, from understanding extractables and leachables regulatory requirements to deciding on a suitable test strategy to decoding the results. We will work with you to understand you requirements, address your needs and deliver the required results to support your regulatory submission and protect patients.
As plastics and rubber material experts, we can also offer consultative support to help clients with:
- In-house and on-site training
- Material selection and failure analysis
- Independent extractables and leachables data review


